
Education
- IRIS Staging System
- Risk Factors for CKD
- Early Diagnosis of CKD
- Creatinine (Dog)
- Urine Collection
- Urine Specific
Gravity - Proteinuria
- Hypertension
- GFR
- GFR in Practice
- Preventing Aminoglycoside-induced AKI
- Treatment of
Vomiting, Nausea and Inappetence in Cats with Chronic Kidney Disease - Diets for Cats with Chronic Kidney Disease
- Reassessment of "Normal" Values in Dogs and Cats with Chronic Kidney Disease
- Differentiation
between Acute Kidney Injury and Chronic Kidney Disease - Recent advances in Mineral and Bone Disorders in CKD
- Utility of Creatinine, UPC, and SDMA in the Early Diagnosis of CKD
- Inhibition of the
renin-angiotensin-aldosterone system in cats and dogs: The emerging role of angiotensin II receptor blockers - What pet owners should know about kidney function and the diagnosis and management of chronic kidney disease in dogs and cats
- Pyelonephritis
- Renal Biomarkers
- Hypercalcemia in
CKD - A review of the role of fibroblast growth factor 23
Hypercalcemia in chronic kidney disease (updated 2022)
Henk Van den Broek, Department of Comparative Biomedical Sciences, Royal Veterinary College, London UK NW1 0TU (hvandenbroek@rvc.ac.uk)
The kidneys play a central role in calcium homeostasis, so unsurprisingly disorders of calcium have been associated with chronic kidney disease (CKD) in dogs and cats. Hypercalcemia based on serum total calcium (total hypercalcemia) was observed in 9-22% of dogs1-3 and in 12-21%4,5 of cats with CKD. In both of these species the occurrence of total hypercalcemia was associated with more severe disease.2,4,6 About half of the serum total calcium concentration consists of hydrated ionized calcium ions, and the other half of protein-bound calcium and a small portion of complexed calcium.7 Whilst the mean total calcium concentration generally does not differ among the different stages of CKD in dogs and cats, mean ionized calcium concentration tends to be lower in animals with IRIS CKD Stage 42,4 Even so, hypercalcemia based on serum ionized calcium (ionized hypercalcemia) has been reported in up to 9% of dogs and 30% of cats with CKD.1-5 Calcium disorders form part of chronic kidney disease-mineral and bone disorder (CKD-MBD), a syndrome consisting of mineral disturbances, bone pathology, and soft tissue calcification.8
Only ionized hypercalcemia is likely to impact health, because the ionized calcium fraction is biologically active.9,10 Hypercalcemia due to CKD is most often not severe and owners may not notice clinical signs.9 Nonetheless, hypercalcemia may worsen signs related to CKD as it further reduces glomerular filtration rate (GFR) and can cause anorexia, polyuria and polydipsia, muscle weakness, and constipation.9,11-13 Hypercalcemia can furthermore contribute to urolithiasis11 and, especially in combination with hyperphosphatemia, to soft tissue calcification.14-16 Calcification of the aorta, gastric wall, kidneys, and paws has been reported in cats with CKD.14,17-20 In men with non-dialysis dependent CKD, increased serum calcium concentrations are associated with increased mortality,21 possibly due to vascular calcification which is an important cause of death in human CKD.22 Higher baseline total calcium concentration was not associated with mortality in cats with CKD,23 but under-diagnosis of ionized hypercalcemia may have affected these results. Furthermore, increasing total calcium over time was associated with evidence of progressive CKD in a recent retrospective study (24 Tang et al., 2021). Nevertheless, the effect of hypercalcemia on prognosis in cats with CKD remains to be fully determined in prospective studies.
Calcium regulation
Serum ionized calcium concentration is regulated by the calcium-sensing receptor and multiple hormones, most importantly parathyroid hormone (PTH) and 1,25(OH)2D3 (calcitriol), via an interplay of these hormones on the physiology of the gut, bone, and kidneys.9,25 Calcitriol stimulates intestinal calcium absorption, whilst PTH increases the extracellular calcium concentration by stimulating tubular calcium reabsorption and osteoclastic bone resorption. Changes in serum calcium concentration can be buffered by storage or release of calcium from bone.9,25 Calcitonin is another hormone involved in calcium regulation, and in contrast to PTH it protects against too high serum ionized calcium concentrations.26 Increases in plasma calcitonin concentration in response to both experimentally-induced and naturally occurring hypercalcemia were observed only in some cats,27,i,28 and it could logically be hypothesized that absence of a calcitonin response would predispose cats to the development of ionized hypercalcemia. However, the importance of calcitonin in calcium homeostasis is debatable in adult mammals.29 Although the plasma concentrations of calcitonin and calcium appeared to change in parallel over time within individual cats that did show a response, this response was heterogeneous between cats.28 Moreover, no clear difference in severity of hypercalcemia was observed between groups of cats that did and did not show an increase in calcitonin in response to hypercalcemia.27,28 Calcitonin therefore only appears to play a minor role in calcium regulation in adult cats.Does CKD cause hypercalcemia?
A concurrent diagnosis of hypercalcemia and azotemia can be a diagnostic challenge for the veterinarian as to the cause and effect relationship. Renal dysfunction could result in hypercalcemia through mechanisms such as reduced urinary excretion of calcium or increased bone turnover,30 whilst ionized hypercalcemia could cause a decrease in GFR and predispose to nephrocalcinosis, thus resulting in renal azotemia.12,13 We have shown that a diagnosis of CKD is a significant risk factor for the development of hypercalcemia in cats, at least based on total calcium.6 Cats with a diagnosis of azotemic CKD were 4 times more likely to develop total hypercalcemia than non-azotemic cats, suggesting that renal dysfunction, or possibly even some management strategies in feline CKD, contribute to a deranged calcium homeostasis.
The pathophysiology of hypercalcemia in CKD is incompletely understood, but several mechanisms may contribute to hypercalcemia in CKD. In dogs with CKD, increases in the complexed calcium fraction predominantly influence serum total calcium concentration.31 A decline in kidney function could therefore contribute to an increase in calcium-containing ionic complexes, and subsequently to total hypercalcemia in this species. The ionized and complexed calcium fractions are freely filtered at the glomerulus, of which 99% is reabsorbed in the renal tubules.25 Decreased glomerular filtration and increased tubular reabsorption are proposed mechanisms by which CKD may contribute to hypercalcemia.30 The reduction in GFR with CKD may contribute to a positive calcium balance if calcium intake exceeds the renal excretory capacity.30,32,33 Tubular reabsorption of calcium could increase due to sustained increases in PTH or inactivating mutations in the calcium-sensing receptor.30 Single nucleotide polymorphisms of the calcium-sensing receptor have been identified in cats,34 and could possibly contribute to increased calcium reabsorption.35 Secondary renal hyperparathyroidism occurs in cats and dogs with CKD,3,4 but hypercalcemia in cats with CKD appears PTH-independent, and physiologically appropriate decreases in plasma PTH concentration in response to hypercalcemia were generally observed in cats.6,11,36
Parathyroid hormone stimulates bone resorption,37 and decreased bone mineral density has been observed in cats and dogs with CKD.38,39 Bone tissue is the most important storage site of calcium in the body, and incapacity of bone to store calcium or increased release of calcium from bone could lead to hypercalcemia.30 Metabolic acidosis is found with increasing frequency as renal function declines,40 and has also been documented to stimulate osteoclastic bone resorption,41 releasing calcium carbonate into the circulation.42 An increased requirement for skeletal buffering of acid could therefore be related to hypercalcemia. Metabolic acidosis is associated with increased urinary calcium excretion in dogs,43,44 but serum ionized calcium concentration does not appear to be affected.45 However, ionized hypercalcemia could develop if renal calcium excretion is impaired.
Do renal diets cause hypercalcemia in cats?
There is debate as to whether clinical renal diets contribute to the development of hypercalcemia in cats. These diets are the mainstay in the management of CKD in cats and dogs and their beneficial effects on quality of life, progression and survival has been proven by multiple studies.36,46-49 Although hypercalcemia and CKD have been diagnosed concurrently (in cats yet to be fed a clinical renal diet),4 hypercalcemia has also been reported to develop in some cats relatively soon after prescription of a clinical renal diet.6,36 In a retrospective study we observed the development of total hypercalcemia in 60 of 191 cats at a median of 175 days after diagnosis of azotemic CKD.6 Although for all cats in that study transition onto a renal diet was advised, no information was available on what owners actually fed these cats. In a smaller prospective study, 2 of 15 cats developed ionized hypercalcemia within 6 months after transition onto a clinical renal diet, and withdrawal of the diet restored normocalcaemia in both cases.36 Clinical renal diets most importantly are restricted in phosphate and protein, but these diets also have lower sodium content, higher potassium content, and alkalinizing properties.50 All these factors could affect calcium homeostasis. Low dietary phosphate or a higher dietary calcium to phosphorus ratio could possibly lead to greater intestinal calcium absorption,16,51 and a positive relationship between intake of purified or hydrolyzed protein and urinary calcium excretion has been identified in humans.52-55 Thus, the lower protein content of renal diets possibly contributes to a decrease in renal calcium excretion. Potassium is supplemented in renal diets, and, depending on its accompanying bicarbonate generating organic anion, reduces urinary calcium excretion.56 Sodium, by contrast, stimulates urinary calcium excretion,57 but is reduced in renal diets. Alkali ingestion possibly contributes to bicarbonate-induced increases in tubular calcium reabsorption.44,58 Therefore, the above characteristics of clinical renal diets (although dependent on the exact formulation) could theoretically all contribute to an increased serum calcium concentration in the cat or dog with CKD.
How to assess calcium status?
Hypercalcemia is diagnosed based on serum calcium concentrations exceeding the upper limit of the reference interval specific for the method used, which usually is a serum total calcium concentration >11.5 mg/dL in dogs and >10.5 mg/dL in cats, or an ionized calcium concentration >6 mg/dL in dogs and >5.6 mg/dL in cats.9 Serum total calcium concentration is often used to assess calcium status, but it is best practice to measure the biologically active ionized calcium concentration.7 Total calcium tends to overestimate ionized calcium concentration in dogs with CKD,1 a discordance probably caused by an increase in the complexed calcium fraction due to reduced GFR, resulting in overestimation of true (ionized) hypercalcemia. In contrast, total calcium underestimates the ionized calcium concentration in cats,5,6 a discrepancy probably influenced by acid-base status. It is often stated that cats with CKD typically have total hypercalcemia only, with the ionized calcium concentration within reference interval. 59-61 Although this may be correct in dogs, this assumption seems untrue in cats: the specificity of serum total calcium to detect ionized hypercalcemia in cats with CKD is close to 100%, and almost all cats with total hypercalcemia will have a concurrent ionized hypercalcemia.5,6 The sensitivity, however, is low and (true) ionized hypercalcemia will be under diagnosed in cats when only total calcium is measured. Serum ionized calcium should therefore be measured to allow a correct diagnosis and adequate management in both cats and dogs.
What to do when hypercalcemia is identified?
Unfortunately, the evidence-base for management of hypercalcemia in animals with CKD is small. When persistent and asymptomatic ionized hypercalcemia is identified in a well-hydrated animal, causes of hypercalcemia unrelated to CKD should be investigated. This is particularly true in cats, where CKD is highly prevalent and therefore a common comorbidity.62 In a previous publication on total hypercalcemia in cats, 6 of 33 azotemic hypercalcemic cats had concurrent neoplastic disease.63 Neoplasia is the most common diagnosis in dogs with ionized hypercalcemia, followed by CKD.64 Therefore, thoracic and abdominal radiographs, and abdominal ultrasound should be performed to assess the presence of neoplastic disease. Hypercalcemia in cats is most often of unknown cause (ie. idiopathic hypercalcemia),11 and it is unclear what role pathophysiological mechanisms resulting in idiopathic hypercalcemia play in the development of hypercalcemia in cats with CKD.
Some medications used in the management of CKD-MBD have been associated with hypercalcemia and should be discontinued if this is identified. The use of aluminium-containing phosphate binders has been associated with low bone turnover and hypercalcemia in human CKD patients.65 Use of calcium-containing phosphate binders16,66,67 or vitamin D analogues or metabolites68 also resulted in hypercalcemia in humans with CKD. Both increase the intake of calcium, which could then exceed the impaired capacity of the kidneys to excrete calcium. Therefore, monitoring of serum ionized calcium is recommended if these drugs are used.50 The use of calcitriol is not recommended in cats with CKD.50,69 In dogs, low doses of calcitriol have been shown to prolong survival time in IRIS CKD Stage 3 and 4,70 and hypercalcemia is uncommon.9,70
A diet change is the first step advised for management of cats with mild hypercalcemia.61 When persistent hypercalcemia occurs or is worsening in a cat fed a renal diet, and no other underlying cause has been identified, it is advisable to discontinue feeding 100% renal diet. Either the proportion of renal diet fed on a daily basis could be reduced, or renal diet could be stopped completely. A dietary transition could then be made to a diet less stringently phosphate restricted, such as a senior diet, or to a calcium oxalate prevention diet, which is restricted in calcium. In one retrospective study where this strategy was followed and a diet with higher phosphorus content and lower Ca:P ratio was fed instead of the renal diet, ionised calcium normalised in 8 of the 10 cats that developed hypercalcaemia following introduction of the renal diet (71: Geddes et al., 2021). Similar findings were reported in a second longitudinal observational study employing the same strategy (72: Schauf et al., 2021). Serum calcium and phosphate concentrations should subsequently be monitored to assess the effect of any dietary change: serum ionized calcium concentration should decrease and phosphate concentration should remain within the IRIS stage-specific target range. One should be aware that hypercalcemia associated with CKD is rarely severe and its prognostic implications largely unknown, whilst strong evidence exists for the beneficial effects of feeding a renal diet to animals with CKD.36,46-49 Therefore, this treatment strategy requires close follow-up and should be clearly discussed with the pet owner.
If serum ionized calcium concentration has not improved 6 weeks after diet change, treatment with specific calcium lowering medications such as bisphosphonates (PO alendronate, IV pamidronate) or prednisolone could be instigated. Bisphosphonates as a treatment option for hypercalcemia have only been examined in few animals, but appear safe and reasonably effective in lowering serum calcium concentrations, although hypercalcemia may reoccur after treatment is stopped.73,74 Promoting calciuresis by intravenous fluid therapy together with furosemide administration could lower serum calcium in the emergency setting, although this is unlikely to be necessary with CKD-related hypercalcemia.
Conclusion
In summary, hypercalcemia is commonly associated with CKD in cats and dogs, and can be a diagnostic challenge. Calcium status should be assessed by measuring serum ionized calcium, and total calcium should not be relied on. Multiple mechanisms by which CKD could cause hypercalcemia have been proposed, including reduced urinary excretion of calcium and metabolic acidosis, but underlying causes other than CKD, most importantly neoplasia, should be ruled out. The first treatment option is often a diet change, for which there is some limited evidence in cats (two case series) when hypercalcemia develops following the introduction of a clinical renal diet.
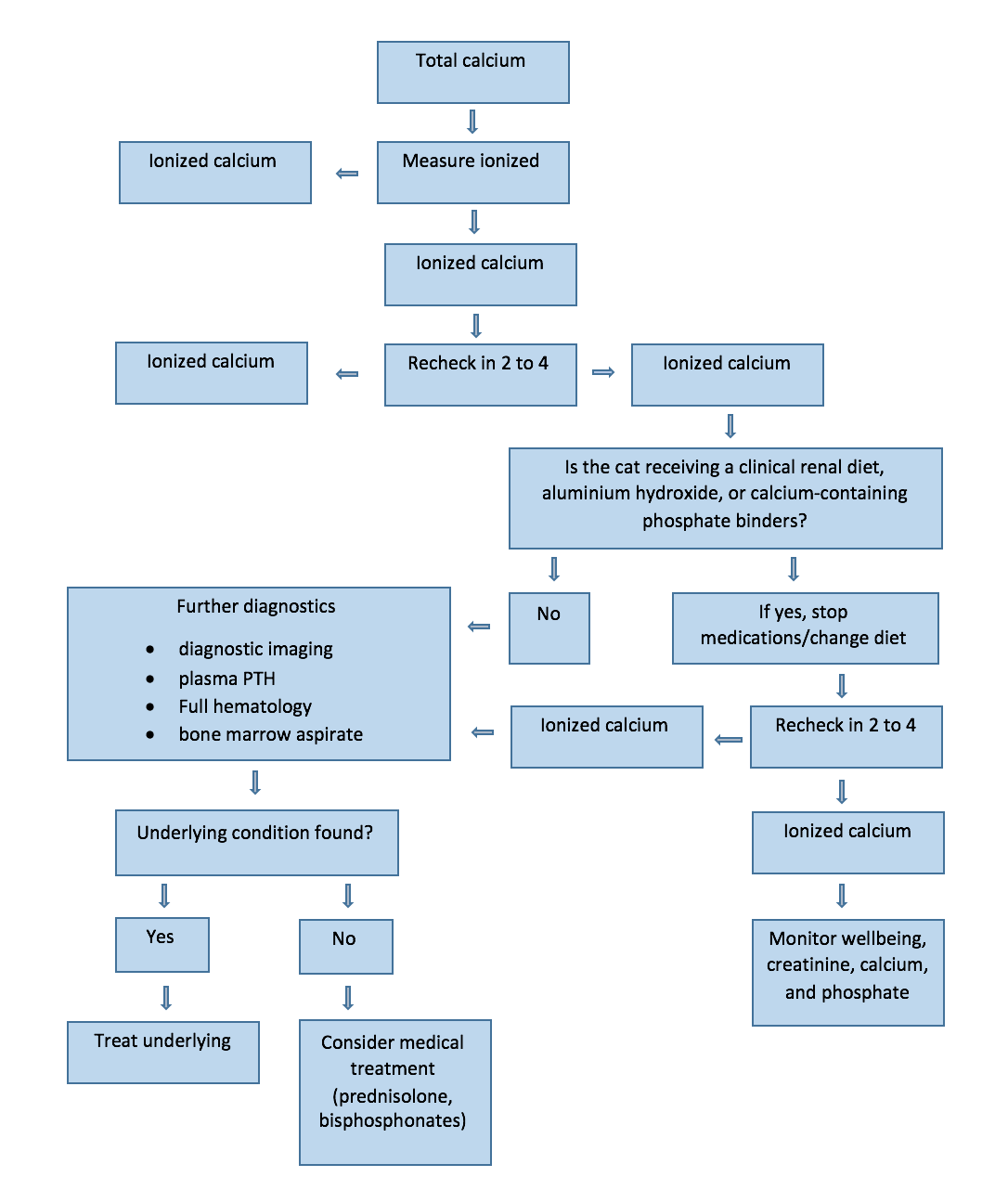
Flow chart for management of hypercalcemia in cats with CKD and mild/asymptomatic hypercalcemia.
References
1. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by use of serum total calcium concentration in dogs. American journal of veterinary research 2005;66:1330-1336.
2. Cortadellas O, Fernandez del Palacio MJ, Talavera J, et al. Calcium and phosphorus homeostasis in dogs with spontaneous chronic kidney disease at different stages of severity. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine 2010;24:73-79.
3. Parker VJ, Harjes LM, Dembek K, et al. Association of Vitamin D Metabolites with Parathyroid Hormone, Fibroblast Growth Factor-23, Calcium, and Phosphorus in Dogs with Various Stages of Chronic Kidney Disease. Journal of veterinary internal medicine 2017;31:791-798.
4. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. The Journal of small animal practice 1998;39:108-116.
5. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire 2010;74:209-213.
6. van den Broek DH, Chang YM, Elliott J, et al. Chronic Kidney Disease in Cats and the Risk of Total Hypercalcemia. Journal of veterinary internal medicine 2017;31:465-475.
7. Schenck PA, Chew DJ. Calcium: total or ionized? The Veterinary clinics of North America Small animal practice 2008;38:497-502, ix.
8. Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945-1953.
9. Schenck PA, Chew DJ, Nagode LA, et al. Chapter 6 - Disorders of Calcium: Hypercalcemia and Hypocalcemia A2 - DiBartola, Stephen P. In: Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice (Fourth Edition). Saint Louis: W.B. Saunders; 2012:120-194.
10. Obi Y, Mehrotra R, Rivara MB, et al. Hidden Hypercalcemia and Mortality Risk in Incident Hemodialysis Patients. The Journal of Clinical Endocrinology & Metabolism 2016;101:2440-2449.
11. Midkiff AM, Chew DJ, Randolph JF, et al. Idiopathic hypercalcemia in cats. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine 2000;14:619-626.
12. Levi M, Ellis MA, Berl T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia. Role of prostaglandins, renin-angiotensin system, and calcium. The Journal of clinical investigation 1983;71:1624-1632.
13. Levi M, Peterson L, Berl T. Mechanism of concentrating defect in hypercalcemia. Role of polydipsia and prostaglandins. Kidney Int 1983;23:489-497.
14. McLeland SM, Lunn KF, Duncan CG, et al. Relationship among serum creatinine, serum gastrin, calcium-phosphorus product, and uremic gastropathy in cats with chronic kidney disease. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine 2014;28:827-837.
15. Shroff RC, McNair R, Skepper JN, et al. Chronic Mineral Dysregulation Promotes Vascular Smooth Muscle Cell Adaptation and Extracellular Matrix Calcification. Journal of the American Society of Nephrology 2010;21:103-112.
16. Block GA, Wheeler DC, Persky MS, et al. Effects of Phosphate Binders in Moderate CKD. Journal of the American Society of Nephrology 2012;23:1407-1415.
17. Barber PJ. Parathyroid gland function in the ageing cat [PhD Thesis]. In: Royal Veterinary College. London: University of London; 1998:289.
18. Chakrabarti S, Syme HM, Brown CA, et al. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Veterinary pathology 2013;50:147-155.
19. Bertazzolo W, Toscani L, Calcaterra S, et al. Clinicopathological findings in five cats with paw calcification. Journal of feline medicine and surgery 2003;5:11-17.
20. Jackson HA, Barber PJ. Resolution of metastatic calcification in the paws of a cat with successful dietary management of renal hyperparathyroidism. The Journal of small animal practice 1998;39:495-497.
21. Kovesdy CP, Kuchmak O, Lu JL, et al. Outcomes associated with serum calcium level in men with non-dialysis-dependent chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN 2010;5:468-476.
22. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. American journal of kidney diseases : the official journal of the National Kidney Foundation 1998;32:S112-119.
23. Geddes RF, Elliott J, Syme HM. Relationship between Plasma Fibroblast Growth Factor-23 Concentration and Survival Time in Cats with Chronic Kidney Disease. Journal of veterinary internal medicine 2015;29:1494-1501.
24. Tang PK, Geddes RF, Chang YM, Jepson RE, Bijsmans E, Elliott J. Risk factors associated with disturbances of calcium homeostasis after initiation of a phosphate-restricted diet in cats with chronic kidney disease. J Vet Intern Med. 2021; 35(1):321-332.
25. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clinical journal of the American Society of Nephrology : CJASN 2015;10:1257-1272.
26. Felsenfeld AJ, Levine BS. Calcitonin, the forgotten hormone: does it deserve to be forgotten? Clinical kidney journal 2015;8:180-187.
27. Pineda C, Aguilera-Tejero E, Raya AI, et al. Assessment of calcitonin response to experimentally induced hypercalcemia in cats. American journal of veterinary research 2013;74:1514-1521.
28 Van den Broek DHN, Geddes RF, Williams TL, Chang Y-M, Jepson RE, Elliott J. Calcitonin response to naturally occurring ionized hypercalcemia in cats with chronic kidney disease. J Vet Intern Med 2018 32(2):727-735.
29. Hirsch PF, Baruch H. Is calcitonin an important physiological substance? Endocrine 2003;21:201-208.
30. Peacock M. Calcium metabolism in health and disease. Clinical journal of the American Society of Nephrology : CJASN 2010;5 Suppl 1:S23-30.
31. Schenck PA, Chew DJ. Determination of calcium fractionation in dogs with chronic renal failure. American journal of veterinary research 2003;64:1181-1184.
32. Pieper AK, Haffner D, Hoppe B, et al. A randomized crossover trial comparing sevelamer with calcium acetate in children with CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation 2006;47:625-635.
33. Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney International 2012;81:1116-1122.
34. Gal A, Ridge TK, Graves TK. Cloning and sequencing of the calcium-sensing receptor from the feline parathyroid gland. Domestic animal endocrinology 2010;38:57-61.
35. Eren PA, Turan K, Berber I, et al. The clinical significance of parathyroid tissue calcium sensing receptor gene polymorphisms and expression levels in end-stage renal disease patients. Clinical nephrology 2009;72:114-121.
36. Barber PJ, Rawlings JM, Markwell PJ, et al. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. The Journal of small animal practice 1999;40:62-70.
37. Chambers TJ, Fuller K, McSheehy PM, et al. The effects of calcium regulating hormones on bone resorption by isolated human osteoclastoma cells. The Journal of pathology 1985;145:297-305.
38. Shipov A, Shahar R, Sugar N, et al. The Influence of Chronic Kidney Disease on the Structural and Mechanical Properties of Canine Bone. Journal of veterinary internal medicine 2018;32(1):280-287.
39. Shipov A, Segev G, Meltzer H, et al. The effect of naturally occurring chronic kidney disease on the micro-structural and mechanical properties of bone. PloS one 2014;9:e110057.
40. Elliott J, Syme HM, Reubens E, et al. Assessment of acid-base status of cats with naturally occurring chronic renal failure. The Journal of small animal practice 2003;44:65-70.
41. Meghji S, Morrison MS, Henderson B, et al. pH dependence of bone resorption: mouse calvarial osteoclasts are activated by acidosis. American journal of physiology Endocrinology and metabolism 2001;280:E112-119.
42. Bushinsky DA, Lechleider RJ. Mechanism of proton-induced bone calcium release: calcium carbonate-dissolution. The American journal of physiology 1987;253:F998-1005.
43. Sutton RA, Wong NL, Dirks JH. Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int 1979;15:520-533.
44. Marone CC, Wong NL, Sutton RA, et al. Effects of metabolic alkalosis on calcium excretion in the conscious dog. The Journal of laboratory and clinical medicine 1983;101:264-273.
45. Kogika MM, Lustoza MD, Notomi MK, et al. Serum ionized calcium in dogs with chronic renal failure and metabolic acidosis. Veterinary clinical pathology / American Society for Veterinary Clinical Pathology 2006;35:441-445.
46. Elliott J, Rawlings JM, Markwell PJ, et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. The Journal of small animal practice 2000;41:235-242.
47. Jacob F, Polzin DJ, Osborne CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in dogs. Journal of the American Veterinary Medical Association 2002;220:1163-1170.
48. Plantinga EA, Everts H, Kastelein AM, et al. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. The Veterinary record 2005;157:185-187.
49. Ross SJ, Osborne CA, Kirk CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. Journal of the American Veterinary Medical Association 2006;229:949-957.
50. Sparkes AH, Caney S, Chalhoub S, et al. ISFM Consensus Guidelines on the Diagnosis and Management of Feline Chronic Kidney Disease. Journal of feline medicine and surgery 2016;18:219-239.
51. Lee K-J, Kim K-S, Kim H-N, et al. Association between dietary calcium and phosphorus intakes, dietary calcium/phosphorus ratio and bone mass in the Korean population. Nutrition Journal 2014;13:114.
52. Allen LH, Bartlett RS, Block GD. Reduction of renal calcium reabsorption in man by consumption of dietary protein. The Journal of nutrition 1979;109:1345-1350.
53. Allen LH, Oddoye EA, Margen S. Protein-induced hypercalciuria: a longer term study. The American journal of clinical nutrition 1979;32:741-749.
54. Schuette SA, Zemel MB, Linkswiler HM. Studies on the mechanism of protein-induced hypercalciuria in older men and women. The Journal of nutrition 1980;110:305-315.
55. Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. The American journal of clinical nutrition 2003;78:584S-592S.
56. Rafferty K, Davies KM, Heaney RP. Potassium intake and the calcium economy. Journal of the American College of Nutrition 2005;24:99-106.
57. Nordin BE, Need AG, Morris HA, et al. The nature and significance of the relationship between urinary sodium and urinary calcium in women. The Journal of nutrition 1993;123:1615-1622.
58. Picolos MK, Lavis VR, Orlander PR. Milk-alkali syndrome is a major cause of hypercalcaemia among non-end-stage renal disease (non-ESRD) inpatients. Clinical endocrinology 2005;63:566-576.
59. Schenck PA, Chew DJ. Hypercalcemia: a quick reference. The Veterinary clinics of North America Small animal practice 2008;38:449-453, viii.
60. Schenck PA. Electrolyte Disorders: Ca-P and Mg. In: Ettinger SJ, Feldman E.C., ed. Textbook of veterinary internal medicine: diseases of the dog and the cat St. Louis, Missouri, USA: Saunders Elsevier; 2010:308.
61. Finch NC. Hypercalcaemia in cats: The complexities of calcium regulation and associated clinical challenges. Journal of feline medicine and surgery 2016;18:387-399.
62. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. Journal of feline medicine and surgery 2014;16:465-472.
3. Savary KC, Price GS, Vaden SL. Hypercalcemia in cats: a retrospective study of 71 cases (1991-1997). Journal of veterinary internal medicine / American College of Veterinary Internal Medicine 2000;14:184-189.
64. Messinger JS, Windham WR, Ward CR. Ionized hypercalcemia in dogs: a retrospective study of 109 cases (1998-2003). Journal of veterinary internal medicine / American College of Veterinary Internal Medicine 2009;23:514-519.
65. Piraino B, Chen T, Puschett JB. Elevated bone aluminum and suppressed parathyroid hormone levels in hypercalcemic dialysis patients. American journal of nephrology 1989;9:190-197.
66. Hill KM, Martin BR, Wastney ME, et al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3--4 chronic kidney disease. Kidney International 2013;83:959-966.
67. Jamal SA, Vandermeer B, Raggi P, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. The Lancet 2013;382:1268-1277.
68. Sprague SM, Llach F, Amdahl M, et al. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney International 2003;63:1483-1490.
69. Hostutler RA, DiBartola SP, Chew DJ, et al. Comparison of the effects of daily and intermittent-dose calcitriol on serum parathyroid hormone and ionized calcium concentrations in normal cats and cats with chronic renal failure. Journal of Veterinary Internal Medicine 2006;20:1307-1313.
70. D. Polzin SR, C. Osborne, J. Lulich, L. Swanson. Clinical benefit of calcitriol in canine chronic kidney disease Journal of veterinary Internal Medicine 2005;19:433.
71. Geddes RF, van den Broek DHN, Chang YM, Biourge V, Elliott J, Jepson RE. The effect of attenuating dietary phosphate restriction on blood ionized calcium concentrations in cats with chronic kidney disease and ionized hypercalcemia. J Vet Intern Med. 2021 Mar;35(2):997-1007.
72. Schauf S, Coltherd JC, Atwal J, Gilham M, Carvell-Miller LJ, Renfrew H, Elliott J, Elliott D, Bijsmans ES, Biourge VC, Watson P, Bakke AM. Clinical progression of cats with early-stage chronic kidney disease fed diets with varying protein and phosphorus contents and calcium to phosphorus ratios. J Vet Intern Med. 2021; 35(6):2797-2811.
73. Hardy BT, de Brito Galvao JF, Green TA, et al. Treatment of ionized hypercalcemia in 12 cats (2006-2008) using PO-administered alendronate. Journal of veterinary internal medicine 2015;29:200-206.
74. Hostutler RA, Chew DJ, Jaeger JQ, et al. Uses and effectiveness of pamidronate disodium for treatment of dogs and cats with hypercalcemia. Journal of veterinary internal medicine 2005;19:29-33. iManiaki E, Pineda C, Dunbar Z, Finch N. Calcitonin response to naturally occurring hypercalcaemia in cats. J Small Anim Pract 2016; 57(Suppl. 1): 32-33 (abstract).