
Education
- IRIS Staging System
- Risk Factors for CKD
- Early Diagnosis of CKD
- Creatinine (Dog)
- Urine Collection
- Urine Specific
Gravity - Proteinuria
- Hypertension
- GFR
- GFR in Practice
- Preventing Aminoglycoside-induced AKI
- Treatment of
Vomiting, Nausea and Inappetence in Cats with Chronic Kidney Disease - Diets for Cats with Chronic Kidney Disease
- Reassessment of "Normal" Values in Dogs and Cats with Chronic Kidney Disease
- Differentiation
between Acute Kidney Injury and Chronic Kidney Disease - Recent advances in Mineral and Bone Disorders in CKD
- Utility of Creatinine, UPC, and SDMA in the Early Diagnosis of CKD
- Inhibition of the
renin-angiotensin-aldosterone system in cats and dogs: The emerging role of angiotensin II receptor blockers - What pet owners should know about kidney function and the diagnosis and management of chronic kidney disease in dogs and cats
- Pyelonephritis
- Renal Biomarkers
- Hypercalcemia in
CKD - A review of the role of fibroblast growth factor 23
GFR
Glomerular filtration rate in dogs and cats (2013)
R Heiene, Oslo, Norway and HP Lefebvre, Toulouse, France
Introduction
Glomerular filtration rate (GFR) is considered the single most useful and sensitive test of renal function.1 Any decrease in GFR generally means that kidney disease is occurring or progressing. Assessment of GFR is therefore pivotal for evaluating severity and progression of renal diseases, especially chronic kidney diseases (CKD). In small animal medicine, the staging system proposed by the International Renal Interest Society (IRIS) is currently based on the concentration of creatinine in blood, but the IRIS board considers that, in the future, GFR measurement could become the major criterion for staging, as it is in humans.2 Several methods for measuring GFR have now been validated in dogs and cats, including plasma clearance techniques using non-radioactive markers such as creatinine and iohexol, that are easier to use than urinary clearance methods. These plasma clearance methods make determination of GFR practical in both clinical and research settings.3 Here we review some recent developments and current challenges regarding plasma clearance methods to assess GFR.
Blood sampling procedure and strategy
Plasma clearance methods require repeated blood samples over a period of several hours following intravenous administration of a suitable marker. Clearance (Cl) is then calculated using the formula Cl = GFR = dose/AUC, where AUC is the area under the plasma disappearance curve of the marker.
Fig 1a: Example of a plasma disappearance curve, where the elimination is monoexponential after an initial "distribution phase" of the marker
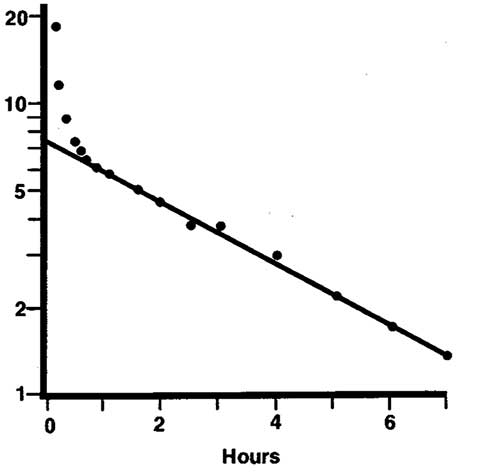
The greater the number of blood samples and the longer the period of collection, the more accurate will be the estimation of the AUC, and thus of the GFR. Sampling should continue ideally until all marker is excreted, but testing is easier if the number of samples and the sampling period are limited. Much research has focussed on determining the optimal number of samples and sampling times to provide suitable limited-sample strategies.
Repeated collection of 5-mL blood samples in cats or miniature dogs may be difficult and lead to excessive blood loss. The total volume of blood collected can be reduced if the assay requires very small volumes of plasma or serum. For example, 0.2 mL of blood is sufficient for blood creatinine assays using an enzymatic method. Paediatric devices based on a capillary system have been proposed as alternatives to vacuum tubes for sampling for these tests.4 Potential advantages include absence of vein collapse, limited blood withdrawal and improved safety.
The timing of blood sampling should be aimed to minimize the proportion of the AUC that is extrapolated.1 For this, the last sampling is especially critical, because it determines the percentage of extrapolated area, which is calculated from the slope of the elimination phase and the last measured concentration. A slight error in calculating the slope may also induce an error in calculating clearance. Ideally, the extrapolated area should be < 20% of the total AUC. The lower the renal function, the slower the elimination of the marker.
Fig1b: Example of how plasma disappearance curves have large or small areas under the curve (AUC) if the marker is slowly (red) or rapidly (green) excreted - reflecting low or high renal function.
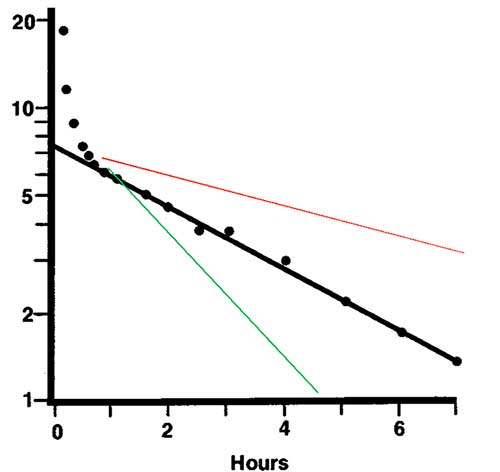
Unfortunately, in clinical practice the GFR of the patient is unknown and the optimum final blood sampling time is therefore uncertain. For most markers this is a problem only if the GFR is very low, in which case blood creatinine is increased. Late sampling can then be planned for.
Creatinine has 2–3 times longer elimination half life than most other markers due to a larger volume of distribution. Blood sampling at 1 and 10 h post-administration provides a relatively accurate estimation of the AUC, with a maximum error of –14% compared to the AUC determined from an 11-point kinetic profile. However, sampling at inappropriate times can lead to errors exceeding 400%.6 It may be an option to keep the dog in the clinic overnight for a 24 hour sample, if the creatinine in the samples can be analyzed directly and it is observed that blood creatinine remains high during the day of sampling.
Correction formulae derived from those used in human medicine (e.g. Brøchner-Mortensen formula) have been proposed and applied to dogs and cats to estimate the actual GFR value from the clearance determined with a limited sampling strategy.5–10
Repeated GFR measurements
A potential problem with repeated GFR measurements in patients is within-individual and between-day variability (i.e. the reproducibility of measurement). If a dog is tested on two separate occasions, what minimum difference between the two GFR findings is needed to exceed physiological and analytical variability, and therefore be interpretable as clinically relevant? Studies using healthy or diseased animals have provided between-day coefficients of variation generally <20% for most markers; 11–14 considered to be acceptable.
Repeated clearance measurements in a given patient should be performed using the same technique to avoid misinterpretation due to methodological differences. Differences of 10–20% are often found in clearance of various markers when performed in the same animals and at the same laboratory.8;15–18
Physiological factors of variation
Among dietary factors which can affect renal function, protein intake is the most important, as a protein rich meal may increase GFR. Hydration status is also crucial because dehydration - even if subclinical - will reduce GFR. Thus, animals should be well hydrated but fasted overnight before the test.
Age
In growing Beagle puppies, the mean GFR decreased ~40% (from 4.1 to 2.5 mL/min/kg) between ages of 9 and 27 weeks.19 GFR was 87% higher in 2-month-old Beagle puppies than in 6–9 year-old Beagle dogs.20 These results indicate that a progressive decline in GFR is normal in the growing pup and should not be interpreted as development of CKD. In kittens, the GFR is higher at ages 9 to 19 weeks than in younger or older individuals.21
In adult dogs, aging seemed to have a limited effect, or an effect obscured by other influences, in longitudinal22 and cross-sectional5 studies. For cats, plasma clearance of creatinine, but not of iohexol, was ~25% lower at ages 9–12 years than in those 7–12 months old.14 Another feline study found no correlation between age and plasma clearance of iohexol or creatinine.9 Thus, any decline in renal function with aging seems limited in healthy dogs and cats. However, the potential clinical relevance of GFR decline in aged animals needs further study.
Body weight
GFR is lower in large dogs than in small dogs. In healthy adult dogs, negative correlations have been shown between body weight and GFR, assessed by plasma clearance of creatinine (n = 113)22 and of iohexol (n = 118).5
Also in cats a negative linear relationship was evident between body weight and GFR assessed by the plasma clearance of iohexol with creatinine, although the R² value was low (<20%).9 In domestic shorthair cats, body weight appears to have a slight effect on blood creatinine concentration (mean ± SD values 142 ± 36 and 161 ± 22 µmol/L for body weights <4 kg and >5 kg, respectively).23
The effect of size may initially seem surprising, but it is not unexpected, as a number of species have been shown to have kidney function more closely related to metabolic needs than to body weight.24
Breed
Breed effects on blood creatinine concentrations were recently reported in dogs23 and cats.24 But this does not mean that a breed-dependency of GFR necessarily exists, as endogenous production of creatinine in muscle also affects blood creatinine concentrations. A good example of this is the Greyhound breed, where the higher blood creatinine concentrations found have been shown to be due to higher production of creatinine rather than to lower GFR.25;26
However, breed differences might still influence GFR. In a recent study,27 GFR was lower in German Shepherds than in English Pointers and English Setters (2.5 ± 0.7, vs. 3.5 ± 0.6 and 3.4 ± 0.8 mL/min/kg, respectively). Age was similar in the three breeds but German Shepherds were heavier; thus differences in body weight, a confounding factor in this study, could have contributed to the observed differences in GFR.
Indexation of glomerular filtration rate
Clearance is measured as mL/min. Standardization (indexation; scaling) of GFR values to the body weight allows comparison of the clearance value to reference ranges for healthy animals. GFR in dogs and cats is traditionally expressed in mL/min/kg. A current challenge is to define the most appropriate way to standardize GFR. Body surface area (BSA) has been proposed as the reference for indexing physiological variables and it is routinely used in human medicine. However, it has been recommended that such an indexation for GFR should be abandonned28 and the formulae used to estimate BSA in dogs are probably inaccurate.29 Indexation to extracellular fluid volume (ECFV) has been proposed as an alternative because one of the major roles of the kidney is to regulate body fluid composition.30 Nevertheless, standardization to ECFV did not produce substantial changes in the relationships between GFR estimates and body weight in adult dogs.5 Moreover, with indexation to ECFV, differences between puppies and adult dogs were still observed, but were inversed.20 In an earlier canine study, standardization to body weight, BSA or ECFV was shown to produce quite different results and for some dogs altered the clinical interpretation of the GFR value obtained.10 A similar problem exists in the cat: standardization to BSA resulted in larger between-individual coefficient of variation (36%) than did standardization to body weight (27%) or ECFV (24%).9
Standardization of GFR is also of concern in obese human patients, who are clearly different from lean individuals of similar body weights. The higher the weight, the lower the GFR indexed to BSA. Other ways to index GFR have been tested unsuccessfully. An absolute, non-corrected GFR is currently recommended in obese patients.31 There are no published data regarding indexation of body weight in obese dogs or cats. In children, variation in body shape or constitution causes difficulties with prediction formulae and simplified approaches.32
Further research is needed in order to evaluate the best method for standardization of GFR values in dogs and cats. Such research is not simple because there is no "golden standard" to relate to. Ideally, regression analysis should be used on a large representative population of adult dogs (different breeds, body weights, age and sex), evaluating different methods of standardization. A simplified alternative would be to stratify the canine population into body weight categories and define an “average” cut-off for the GFR estimates in each category. The main limitation then would be that the greater the range of body weights for a given category, the more inaccurate the corresponding derived cut-off values. For the time being, it seems logical to standardize to body weight as most publications on reference intervals make use of this approach.
Reference intervals
Defining GFR reference intervals for dogs and cats is a prerequisite for classification of patients as normal or abnormal. Reference values are only published for adult animals; while values are different for puppies and kittens (see above). It is generally considered that the lower cut-off value is between 1.5 and 2.0 mL/min/kg. Considering the effect of physiological variability observed in recent studies, however, it appears that a unique cut-off value is not acceptable for the overall canine population. Stratification is advisable according to body weight, but possibly not to age.
In one previous study (113 healthy dogs),33 animals were divided into 4 body weight categories (Mini, Medium, Maxi and Giant). The corresponding GFR values (mean ± SD) were 3.7 ± 0.5, 3.0 ± 0.5, 2.5 ± 0.4, and 2.4 ± 0.6 mL/min/kg, respectively (Lefevbre HP et al ; unpublished data). If the hypothetical distributions of GFR values were normal, the corresponding lower limits of the reference ranges could be considered to be 2.7, 2.0, 1.7 and 1.3 mL/min/kg. In another study involving 118 adult healthy dogs, the reference ranges in different weight quartiles were 1.54–4.25, 1.29–3.50, 1,2–3.36, and 1.12–3.39 mL/min/kg, respectively, for body weight quartiles of 1.8–12.4, 13.2–25.5, 25.7–31.6, and 32.0–70.3 kg.5
These results, obtained with two different markers (creatinine and iohexol), demonstrate that depending on body weight, the lower cut-off value is higher for small dogs,and lower for giant dogs. Thus, choosing a single value of 1.5 mL/min/kg to declare a dog as renal-impaired will be too low for small dogs (giving some false negative results) and too high for giant dogs (some false positive results).
Breed may also affect the reference intervals. For example, tentative reference intervals for English Pointers, English Setters and German Shepherds are 2.3–5.1, 1.8–5.0 and 1.7–3.8 mL/min/kg.27 It seems impractical to try to establish breed-specific reference intervals for all breeds, other than the major ones. In our experience, most breeds tend to follow their weight category with respect to reference values in healthy dogs.
Plasma clearance reference ranges for cats have been proposed recently: 1.0–3.5 mL/min/kg for iohexol and 1.3–3.8 mL/min/kg for creatinine.9 From the point of view of allometric relationships,24 it is interesting that the cut-off value for GFR is lower in cats than in dogs of similar body weight.
These preliminary results provide some estimates of reference intervals for veterinary nephrologists, but further investigations in larger canine and feline populations are obviously required.
Perspectives on GFR measurements in cardiovascular and endocrine diseases
GFR measurements may be interesting to assess renal function in small animal patients with non-primary renal diseases, as shown recently for some cardiovascular and endocrine diseases.
Cardiovascular diseases
CKD was shown recently to be prevalent in humans with cardiovascular disease, wherein a very slight decrease in renal function may dramatically increase the cardiovascular risk.34 Azotaemia is common in dogs with cardiac diseases,35 but the degree of azotaemia is mostly mild to moderate and not related to changes in cardiovascular variables.35 Interestingly, the GFR in 24 dogs with chronic valvular disease was significantly lower in NYHA III-IV (1.7±0.7 mL/min/kg) than in NYHA I-II (3.1±0.8 mL/min/kg). Only 1/15 NYHA dogs in class I-II had GFR <2 mL/min/kg and only 2/9 NYHA class III-IV dogs had GFR >2 mL/min/kg.35 Further investigations are needed to identify the cause of decreased GFR and its clinical relevance to the outcome of cardiac disease.
Endocrine diseases
Interactions exist between endocrine systems and kidney function under both physiological and clinical conditions. One of the best examples in veterinary medicine is probably the effect of hyper- or hypothyroidism on renal function in small animals.39
Hyperthyroidism is a common endocrine disorder in aged cats and a 14–40% prevalence of pre-existing CKD has been reported in affected cats.36 Evaluation of kidney function in a hyperthyroid cat is pivotal as hyperthyroidism can mask and sometimes worsen CKD. CKD became clinically apparent after treatment of hyperthyroidism in about 17–39% of cats.36 A challenge in such patients is to predict the risk of post-treatment azotaemia. Pre-treatment GFR was shown to be predictive of development of post-treatment CKD.37;38 An accurate evaluation of kidney function can be performed from 4 weeks after 131I-treatment.38;39
GFR was shown to be decreased in experimentally-induced canine hypothyroidism whereas blood creatinine concentrations remained unaltered.40 In 14 dogs with naturally occurring hypothyroidism, GFR was <2 mL/min/kg in all, and increased with levothyroxine treatment.41 The potential effects of renal dysfunction and development of subclinical CKD on the clinical outcome of hypothyroid dogs require further investigation.
Other situations where questions might arise about adequacy of kidney function are in diabetes mellitus or spontaneous hyperadrenocorticism, or during glucocorticosteroid therapy. The usefulness of GFR esitmation is of limited value at present in these situations, because little is know about how they affect GFR, so studies are needed to establish GFR values for these patients.
References
- Heiene R, Lefebvre HP.Assessment of renal function. In: Elliott J and Grauer GF eds. BSAVA manual of canine and feline nephrology and urology. 2 ed. 2007;117–125.
- Elliott J.Staging chronic kidney disease. In: Elliott J and Grauer GF eds. BSAVA manual of canine and feline nephrology and urology. 2 ed. 2007;159–166.
- Lefebvre HP.Glomerular filtration rate in dogs and cats. Proceedings ACVIM Forum 2010; 491-493.
- Reynolds BS, Boudet KG, Faucher MR, Germain C, Geffre A, Lefebvre HP (2007), Comparison of a new device for blood sampling in cats with a vacuum tube collection system - plasma biochemistry, haematology and practical usage assessment, Journal of Feline Medicine and Surgery 9: 382–386
- Bexfield NH, Heiene R, Gerritsen RJ, Risøen U, Eliassen KE, Herrtage MH, Michell AR (2008), Glomerular filtration rate estimated by 3-sample plasma clearance of iohexol in 118 healthy dogs, J.Vet.Intern.Med. 22: 66–73
- Brown SA, Finco DR, Boudinot FD, Wright J, Taver SL, Cooper T (1996), Evaluation of a single injection method, using iohexol, for estimating glomerular filtration rate in cats and dogs, Am.J Vet.Res. 57: 105–110
- Finch NF, Syme H, Peters AM, Gerritsen RJ, Croebels S, Heiene R (2011), A formula to predict glomerular filtration rate (GFR) using plasma clearance of iohexol in cats, J.Vet.Intern.Med. In press:
- Gleadhill A, Michell AR (1996), Evaluation of iohexol as a marker for the clinical measurement of glomerular filtration rate in dogs, Res.Vet.Sci. 60: 117–121
- Heiene R, Reynolds BS, Bexfield NH, Larsen S, Gerritsen RJ (201), Estimation of glomerular filtration rate via 2- and 4- sample plasma clearance of iohexol and creatinine in clinically normal cats, American Journal of Veterinary Research 70: 176–185
- Heiene R, Moe L (1999), The relationship between some plasma clearance methods for estimation of glomerular filtration rate in dogs with pyometra, J.Vet.Intern.Med. 13: 587–596
- Finco DR, Braselton WE, Cooper TA (2001), Relationship between plasma iohexol clearance and urinary exogenous creatinine clearance in dogs, J.Vet.Intern.Med. 15: 368–373
- Finco DR (2005), Measurement of glomerular filtration rate via urinary clearance of inulin and plasma clearance of technetium Tc 99m pentetate and exogenous creatinine in dogs, Am.J.Vet.Res. 66: 1046–1055
- Miyamoto K (2001), Evaluation of plasma clearance of inulin in clinically normal and partially nephrectomized cats, Am.J.Vet.Res. 62: 1332–1335
- van Hoek I, Vandermeulen E, Duchateau L, Lefebvre HP, Croubels S, Peremans K, Polis I, Daminet S (2007), Comparison and reproducibility of plasma clearance of exogenous creatinine, exo-iohexol, endo-iohexol, and Cr–51-EDTA in young adult and aged healthy cats, Journal of Veterinary Internal Medicine 21: 950–958
- Bird NJ, Peters C, Michell AR, Peters AM (2007), Extracellular distribution volumes of hydrophilic solutes used to measure the glomerular filtration rate: comparison between chromium–51-EDTA and iohexol, Physiol Meas. 28: 223–234
- Florijn KW, Barendregt JNM, Lentjes EGWM, van Dam W, Prodjosudjadi W, van Saase JLCM (1994), Glomerular filtration rate measurement by “single-shot” injection of inulin, Kidney International 46: 252–259
- Frennby B, Sterner G (2002), Contrast media as markers of GFR, Eur.Radiol. 12: 475–484
- Watson AD, Lefebvre HP, Concordet D, Laroute V, Ferre JP, Braun JP, Conchou F, Toutain PL (2002), Plasma exogenous creatinine clearance test in dogs: comparison with other methods and proposed limited sampling strategy, J.Vet.Intern.Med. 16: 22–33
- Lane IF, Shay DH, Burton SA, Donald AW (2000), Quantitative urinalysis in healthy Beagle puppies from 9 to 27 weeks of age, American Journal of Veterinary Research 61: 577–581
- Laroute V, Chetboul V, Roche L, Maurey C, Costes G, Pouchelon JL, De La FF, Boussouf M, Lefebvre HP (2005), Quantitative evaluation of renal function in healthy Beagle puppies and mature dogs, Res.Vet.Sci. 79: 161–167
- Hoskins JD, Turnwald GH, Kearney MT, Gossett KA, Fakier N (1991), Quantitative Urinalysis in Kittens from 4 to 30 Weeks After Birth, American Journal of Veterinary Research 52: 1295–1299
- Queau Y, Biourge V, Germain CBJP, Watson AD, Jeunesse E, Lefebvre HP. Effect of aging on blood creatinine clearance in dogs. J.Vet.Intern.Med. 598. 2007. Ref Type: Abstract
- Reynolds BS, Boudet KG, Germain CA, Braun JPD, Lefebvre HP (2008), Determination of reference intervals for plasma biochemical values in clinically normal adult domestic shorthair cats by use of a dry-slide biochemical analyzer, American Journal of Veterinary Research 69: 471–477
- Edwards NA (1975), Scaling of renal functions in mammals, Comp Biochem.Physiol A 52: 63–66
- Hill RC, Crawford PC, Scott KC, Queau Y, Biourge V, Lefebvre HP (2007), Creatinine disposition and daily production in healthy greyhound dogs, Journal of Veterinary Internal Medicine 21: 274
- Kukanich B, Coetzee JF, Gehring R, Hubin M (2007), Comparative disposition of pharmacologic markers for cytochrome P–450 mediated metabolism, glomerular filtration rate, and extracellular and total body fluid volume of Greyhound and Beagle dogs, Journal of Veterinary Pharmacology and Therapeutics 30: 314–319
- Seguela J, Queau Y, Murgier P, Mimouni P, Concordet D, Duperron C, Lefebvre HP. Glomerular filtration rate in healthy English Pointer, English Setter and German Shepherd Dogs. J.Vet.Intern.Med. 764. 2009. Ref Type: Abstract
- Turner ST, Reilly SL (1995), Fallacy of Indexing Renal and Systemic Hemodynamic Measurements for Body-Surface Area, American Journal of Physiology-Regulatory Integrative and Comparative Physiology 268: R978-R988
- Price GS, Frazier DL (1998), Use of body surface area (BSA)-based dosages to calculate chemotherapeutic drug dose in dogs: I. Potential problems with current BSA formulae, Journal of Veterinary Internal Medicine 12: 267–271
- Peters AM (1992), Expressing glomerular filtration rate in terms of extracellular fluid volume, Nephrology Dialysis Transplantation 7: 205–210
- Delanaye P, Radermecker RP, Rorive M, Depas G, Krzesinski JM (2005), Indexing glomerular filtration rate for body surface area in obese patients is misleading: concept and example, Nephrology Dialysis Transplantation 20: 2024–2028
- Hjorth L, Wiebe T, Karpman D (2002), Correct evaluation of renal glomerular filtration rate requires clearance assays, Pediatr.Nephrol. 17: 847–851
- Lefebvre HP, Jeunesse E, Concordet D, Ferre JP, De la Farge F, Laroute V, Giraudel J, Watson AD. Assessment of glomerular filtration rate using plasma exogenous creatinine clearance test: preliminary results in a healthy canine population. J.Vet.Intern.Med. 415. 2004. Ref Type: Abstract
- Ritz E, McClellan WM (2004), Overview: Increased cardiovascular risk in patients with minor renal dysfunction: An emerging issue with far-reaching consequences, Journal of the American Society of Nephrology 15: 513–516
- Nicolle AP, Chetboul V, Allerheiligen T, Pouchelon JL, Gouni V, Tessier-Vetzel D, Sampedrano CC, Lefebvre HP (2007), Azotemia and glomerular filtration rate in dogs with chronic valvular disease, Journal of Veterinary Internal Medicine 21: 943–949
- van Hoek I, Daminet S (2009), Interactions between thyroid and kidney function in pathological conditions of these organ systems: A review, General and Comparative Endocrinology 160: 205–215
- Becker TJ, Graves TK, Kruger JM, Braselton WE, Nachreiner RF (2000), Effects of methimazole on renal function in cats with hyperthyroidism, Journal of the American Animal Hospital Association 36: 215–223
- van Hoek I, Lefebvre HP, Peremans K, Meyer E, Croubels S, Vandermeulen E, Kooistra H, Saunders JH, Binst D, Daminet S (2009), Short- and long-term follow-up of glomerular and tubular renal markers of kidney function in hyperthyroid cats after treatment with radioiodine, Domestic Animal Endocrinology 36: 45–56
- Boag AK, Neiger R, Slater L, Stevens KB, Haller M, Church DB (2007), Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine, Veterinary Record 161: 711–715
- Panceira DL, Lefevbre HL (2009). Effect of Experimental Hypothyroidism on Glomerular Filtration Rate and blood creatinine Concentration in Dogs. J.Vet.Intern.Med. 1045–1050.
- Gommeren K, van Hoek I, Lefebvre HP, Benchekroun G, Smets P, Daminet S (2009), Effect of Thyroxine Supplementation on Glomerular Filtration Rate in Hypothyroid Dogs, Journal of Veterinary Internal Medicine 23: 844–849