
Education
- IRIS Staging System
- Risk Factors for CKD
- Early Diagnosis of CKD
- Creatinine (Dog)
- Urine Collection
- Urine Specific
Gravity - Proteinuria
- Hypertension
- GFR
- GFR in Practice
- Preventing Aminoglycoside-induced AKI
- Treatment of
Vomiting, Nausea and Inappetence in Cats with Chronic Kidney Disease - Diets for Cats with Chronic Kidney Disease
- Reassessment of "Normal" Values in Dogs and Cats with Chronic Kidney Disease
- Differentiation
between Acute Kidney Injury and Chronic Kidney Disease - Recent advances in Mineral and Bone Disorders in CKD
- Utility of Creatinine, UPC, and SDMA in the Early Diagnosis of CKD
- Inhibition of the
renin-angiotensin-aldosterone system in cats and dogs: The emerging role of angiotensin II receptor blockers - What pet owners should know about kidney function and the diagnosis and management of chronic kidney disease in dogs and cats
- Pyelonephritis
- Renal Biomarkers
- Hypercalcemia in
CKD - A review of the role of fibroblast growth factor 23
A review of the role of fibroblast growth factor 23 in phosphate homeostasis and the pathophysiology of mineral bone disorder associated with chronic kidney disease
Jonathan Elliott1, Rebecca Geddes2, Rosanne Jepson2
Departments of Comparative Biomedical Sciences1 and Clinical Sciences and Services2 Royal Veterinary College, University of London
Summary
This article provides a comprehensive review of the current state of knowledge of fibroblast growth factor 23 (FGF23) physiology and pathophysiology. Sections 1 to 3 deal with the physiology of phosphate homeostasis and how FGF23 integrates into the systems regulating both phosphate and calcium. Section 4 explores the role of FGF23 in the pathophysiology of mineral bone disturbance in chronic kidney disease. Sections 5 and 6 provide the evidence which underpins the clinical utility of measuring serum FGF23 (now commercially available) in the management of the different stages of CKD. These recommendations have been incorporated into the IRIS 2023 treatment guidelines for cats with CKD and are summarised in Figures 1 and 2 which accompany this review. Section 7 sets out the adverse renal and extra-renal effects of very high levels of FGF23 and how these might contribute to both progressive kidney injury and the uraemic syndrome evident in later stage CKD.
1. Introduction
Despite the fact that phosphorus is a major element in mammals, the systems regulating its homeostasis remain to be fully determined. Inability to regulate serum phosphate because of chronic kidney disease (CKD) leads to secondary renal hyperparathyroidism and primary disorders of the parathyroid gland lead to disturbances in circulating concentrations of phosphate ions. In the 1980s, therefore, parathyroid hormone and calcitriol were thought to be involved in phosphate regulation. Nevertheless, it was recognised that the primary role of these two hormones was regulation of calcium whereas phosphate regulation was of secondary importance. Thus, the search for a hormone whose primary function was to regulate phosphorus, a so called ‘phosphotonin’, continued.
As with many endocrine systems, clues as to the phosphate regulating system came from endocrine disorders that led to over-activity of the hormone. Phosphate wasting diseases which were not accompanied by hypercalcaemia (as would be the case in primary hyperparathyroidism), were identified in human patients and genetic analysis led to the discovery of two distinct disorders, namely X-linked hypophosphataemic rickets (The HYP Consortium 1995) and autosomal dominant hypophosphataemic rickets (ADHR; The ADHR Consortium, 2000). The former was eventually characterised as an inactivating mutation of the Phosphate Regulating Endopeptidase X-Linked (PHEX) gene encoding an enzyme (endopeptidase) responsible for FGF23 metabolism. The latter, led to the discovery of FGF23, a new FGF family member, mutations of which were postulated to be activated in patients with ADHR.
Concomitant discovery of the anti-ageing gene, klotho in 1997 (Kuro-o et al., 1997) demonstrated that the premature ageing phenotype caused by knocking out this gene was associated with hyperphosphataemia, premature osteoporosis and ectopic calcification. Similar phenotypes were observed when FGF23 was knocked out in mice and this led to the realisation that FGF23 and klotho function on a common signalling pathway, working together to regulate phosphate with klotho being an essential co-factor (co-receptor) for FGF23 to activate the FGF-1 receptor (Kurosu et al., 2006). These seminal discoveries underpin our current knowledge of the FGF23-klotho axis in phosphate homeostasis and the adaptations and maladaptation which occur in CKD described below.
2. Site of production of FGF23 and principal regulators of FGF23 synthesis and secretion
Calcium and phosphate are major elements that constitute the endoskeleton. One of the features that enabled successful evolution to terrestrial life was the development of an endoskeleton and with it, the means of maintaining the essential elements of calcium and phosphate in solution in extracellular fluids bathing soft tissues whilst also facilitating their deposition within bone and teeth. One of the adaptations that permits this is the formation of calciprotein particles (CPPs) within blood which allow complexes of calcium and phosphate ions to exist in colloidal form bound to proteins such as fetuin A, matrix gla protein and gla rich protein (see Smith et al., 2020). The particles form first as monomers (calcium and phosphate ion complexes bound to fetuin A which are c10 nm in diameter) and then as primary particles (30 to 100 nm in diameter) which are taken up at sites of bone turnover and are scavenged by Kupfer cells in the liver. Primary particles increase in the blood following a meal and act as a buffer to rapidly absorbed calcium and phosphate limiting changes in free concentration of these ions (Tiong et al., 2022). Primary particles can mature into secondary calciprotein particles (100-250 nm; spikey in shape, pro-inflammatory and linked to vascular calcification) under circumstances where phosphate is present in excess, there is low serum magnesium or fetuin A or low vitamin K (needed for post-translational modification of matrix gla protein and gla rich protein) (see Smith et al., 2020).
It seems logical, therefore, that bone will be integrally involved in sensing of phosphate and in synthesis and secretion of the phosphate regulating hormone. Osteocytes are the main site of production of FGF23, a 251 amino acid protein hormone. The way in which bone cells sense phosphate ions and respond by increasing synthesis and secretion of FGF23 has proved difficult to determine (see Vervolet 2022) and remains to be fully understood. Hypophosphataemia does not fully suppress FGF23 secretion so phosphate ions, whilst being an important stimulus are not the only factor influencing FGF23 synthesis and secretion. There appears to be a significant delay between increased dietary phosphate intake and a rise in FGF23 secretion by bone of up to 24 hours. This appears to be true in cats where acute phosphate feeding did not increase serum FGF23 concentrations over the first 6 hours post-prandially (Coltherd et al., 2019) whereas intake of the same diets for 2 weeks led to persistently elevated plasma FGF23 concentrations (Alexander et al., 2019). Three main pathways linking phosphate ion concentration to FGF23 secretion have been proposed. These include:
- The activity of the membrane inorganic phosphate transporters in osteocytes PiT-2/ SLC20A2 (Bon et al., 2018)
- The FGF-1 receptor on osteocytes to which phosphate ions bind activating second messenger systems which upregulate the expression of GALAT-3, an enzyme which O-glycosylates the FGF23 molecule making it resistant to inactivating proteases in the Golgi apparatus (Takashi et al., 2019)
- Calciprotein particles which are sensed by osteocytes leading to increased fgf23 gene transcription. Both calcium and phosphate ions contribute to the formation of CPPs and it appears to be the primary form of these particles which regulate FGF23 synthesis (Akiyama et al., 2020).
The delay between increased phosphate ions entering the body via the intestine and a rise in plasma FGF23 occurring seems to be because regulation of FGF23 occurs at the transcriptional and post-translational processing of FGF23 rather than the stored hormone being secreted from storage granules in the osteocytes. The relative importance of each of the three mechanisms listed above is not known. Much of the regulation of FGF23 appears to occur at the level of post-translational processing of the protein in the trans-Golgi network where proteolysis occurs and inactive C-terminal FGF23 is released rather than intact FGF23. Where processed FGF23 is stored prior to secretion is not understood although classical secretory granules do not appear to be involved (Yamamoto et al., 2016). O-glycosylation at a critical cleavage site prevents, and phosphorylation enhances, proteolytic cleavage of the intact molecule and mutations in the enzymes involved can lead to disorders in phosphate homeostasis (Tagliabracci et al., 2014). Thus, it is important to understand which portion of the molecule is being detected by any diagnostic assays used. The intact molecule is the physiologically active form of FGF23.
3. Integration of FGF23 with other hormones regulating calcium and phosphate
Other physiological factors regulating FGF23 synthesis and secretion include calcitriol (active vitamin D3) and PTH, both of which increase plasma FGF23 concentrations (Lavi-Moshayoff et al., 2010; Kolek et al., 2005). The relationship between PTH and FGF23 is complex because the main stimulus for PTH synthesis is a low ionised calcium concentration sensed through the calcium sensing receptor. Secretion of FGF23 requires the plasma ionised calcium concentration to be above a threshold level. Indeed, as plasma ionised calcium rises above that threshold, plasma FGF23 concentrations increase and plasma calcium concentration is an independent factor related to serum FGF23 concentration in many species, including the cat (Geddes et al., 2013). Thus, PTH may only stimulate FGF23 secretion once ionised calcium concentration has returned to a more normal value, another reason why the rise in FGF23 may lag behind any increase in plasma PTH concentration.
The relationship between PTH, calcitriol and FGF23 appears to involve feedback loops such that calcitriol synthesis is activated by PTH, leading to increased calcium and phosphate absorption from the intestine. PTH increases osteoclast activity, releasing calcium and phosphate ions from bone whilst acting on the kidney to conserve calcium ions (distal tubule) and excrete phosphate ions (proximal tubule). Both PTH and calcitriol increase FGF23 secretion which prevents plasma phosphate increasing by further reducing phosphate reabsorption in the proximal tubules (down-regulating NaPi transporter expression; Segawa et al., 2003), inhibiting calcitriol synthesis (Shimada et al., 2004) and inhibiting PTH secretion (Krajisnik et al., 2007), thus preventing further entry of calcium and phosphate into extracellular fluid from the diet and from bone. This illustrates how the bone, intestine and kidney work together to integrate regulation of calcium and phosphate, ensure bone mass is maintained, ionised calcium concentration is tightly regulated and phosphate concentration not allowed to increase persistently to the extent that would lead to mineralisation of soft tissues rather than bone itself.
The full integration of this mineral regulating system is not fully understood. The effects of FGF23 mentioned above (inhibition of PTH synthesis and secretion and inhibition of calcitriol synthesis) plus its action of inhibiting the expression of phosphate transporters in the proximal convoluted tubule (leading to its phosphaturic effect) all appear to require alpha-klotho (the transmembrane protein) to enable FGF23 to activate the FGF-1 receptor through which it mediates these effects. Alpha klotho is cleaved from its transmembrane location to release soluble klotho into the circulation, the physiological importance of which is poorly understood. Both soluble klotho and FGF23 may have effects that are independent of each other, for example, klotho independently upregulated TRPV5 channel expression in the distal tubule of the kidney increasing calcium reabsorption (Wolf et al., 2014). For FGF23, these effects independent of klotho may be more of pathophysiological relevance than physiologically important (see below).
4. Role of FGF23 in Mineral and Bone Disturbance associated with CKD
In human patients with CKD, provided vitamin D status is normal, the first indication of the presence of mineral bone disturbance associated with CKD is an increase in plasma FGF23 concentration (Isakova et al., 2011). This appears to be an adaptive response to whole body phosphate retention where a decline in GFR reduces renal capacity to excrete the same amount of phosphate per day. Inhibition of proximal tubular reabsorption of phosphate allows each remaining functioning nephron to excrete more phosphate and for the fasting plasma phosphate concentration to remain stable on the same diet despite the fall in GFR.
The same pattern of changes in mineral bone disturbance most likely occurs in cats and dogs. We know most about the cat to date where plasma FGF23 was elevated in apparently healthy cats which went on to develop azotaemic CKD within 12 months of screening when compared with cats which remained healthy and non-azotaemic 12 months after screening for CKD (Finch et al., 2013). In dogs, FGF23 also appears to be an early indicator of mineral bone disorder (Harjes et al., 2017) and predicts the development of hyperphosphataemia and progression of CKD (Miyakawa et al., 2021).
Intact FGF23 is a 32 kDa protein and is cleared from plasma by renal excretion. Thus, part of the reason it is elevated in CKD is due to reduced renal clearance. Nevertheless, plasma phosphate is an independent predictor of plasma FGF23 concentrations in cats with CKD. In a study where cats were grouped according to IRIS CKD stage and classified either as hyperphosphataemic or normophosphataemic for their stage based on the stage-related IRIS plasma phosphate targets, those that were hyperphosphataemic for their stage had significantly higher plasma FGF23 concentrations compared to those that were normophosphataemic, despite no difference in creatinine concentrations (Geddes et al., 2013), demonstrating the importance of phosphate in determining circulating intact FGF23 concentration. Other factors that were independently related to plasma FGF23 concentration in this study included plasma total calcium concentration and packed cell volume (which was inversely related – the lower the PCV the higher the plasma FGF23 concentration).
An additional important factor influencing plasma FGF23 concentrations in cats with CKD that was not measured in these early studies is plasma magnesium concentration. Van den Broek et al., (2018) showed that low concentrations of plasma magnesium were associated with higher plasma FGF23 concentrations, an observation that is compatible with the human literature and which suggests that plasma magnesium should be measured in CKD patients when assessing mineral bone disturbance associated with CKD.
Plasma FGF23 at initial diagnosis of CKD appears to be an independent risk factor for all-cause mortality (independent of age and plasma creatinine concentration) and for future progression (>25% increase in plasma creatinine concentration within 12 months) of CKD in cats (Geddes et al., 2015). Similar findings have been reported for dogs on progression of CKD and survival (Miyakawa et al., 2021; Rudinsky et al., 2018). These findings demonstrate the value of plasma FGF23 as a prognostic biomarker in feline and canine CKD. These epidemiological risk factor studies do not prove whether FGF23 is causative of poor outcome or merely a biomarker that is indirectly associated.
Nevertheless, it does suggest that at some point increasing plasma concentrations of FGF23 become a maladaptive response of bone cells to CKD. The point at which transition from an appropriate adaptive response, which enables plasma phosphate regulation to occur despite loss of renal function becomes a maladaptive response, where soft tissue mineralisation occurs with osteodystrophy, remains to be determined in veterinary species. In humans it has been suggested that progression to this maladaptive situation occurs as expression of renal klotho diminishes. Down-regulation of tissue klotho expression occurs in CKD (Sakan et al., 2014) secondary to uraemic toxins (indoxyl sulphate and p-cresyl; Young & Wu, 2012) and proteinuria (Delitsikou et al. 2020). This makes FGF23 much less able to activate FGF-1 receptors (where klotho is needed as a co-receptor) at which point plasma PTH concentrations start to rise (due to lack of FGF23 inhibition of PTH synthesis and secretion) and eventually plasma phosphate concentrations start to increase and ionised calcium concentrations decrease. An independent association of serum concentrations of indoxyl sulphate and FGF23 has been also found in cats (Liao et al., 2019) but assessment of changes in klotho expression in veterinary species remain to be made.
Other pathological factors that may give rise to elevated FGF23 concentrations in plasma in clinical patients which have not been studied in any detail in veterinary patients include chronic inflammation and iron deficiency which may occur together with anaemia in veterinary CKD patients. Iron deficiency leads to increased fgf23 gene expression via HIF1α (the metabolism of which is iron dependent). This results in increased circulating concentrations of c-terminal FGF23 (cFGF23) as post-translational proteolysis prevents secretion of intact FGF23 occurring. However, some of the intravenous iron products used to treat iron deficiency (iron polymaltose and ferric carboxymaltose) block post-translational proteolysis of FGF23 leading to increased plasma intact FGF23. Recombinant erythropoietin increases fgf23 gene expression and serum cFGF23 concentrations and in high doses or when combined with intravenous iron preparations may increase plasma concentrations of intact FGF23 although the clinical importance of this in human medicine is unclear. See Wheeler & Clickenbeard (2019) and Zhang et al. (2021) for recent reviews of this topic.
Cytokines such as TNFα, IL-1β and IL-6 are potent stimuli for FGF23 secretion from osteocytes and have been demonstrated to play a role in upregulating intact FGF23 in vivo in experimental models of both acute kidney injury and chronic kidney disease (Durlarcher-Betzer et al., 2018). The cross-talk between chronic inflammation, iron deficiency and anaemia in CKD is complex and of clinical relevance particularly in the later stages of CKD where, in addition to these factors increasing circulating FGF23 concentrations, pathological levels of FGF23 can be proinflammatory (by activating FGF-4 receptors on macrophages) and exacerbate anaemia by inhibiting erythropoietin production (see Babbit & Sitara 2019).
5. Clinical utility of measurement of FGF23
Intact FGF23 is now offered as a diagnostic assay by commercial laboratories to assist in the monitoring of treatment of CKD. In early CKD where a diagnosis has been made in the absence of an elevated plasma creatinine concentration, the need for dietary phosphate restriction cannot be made by the measurement of serum phosphate. All veterinary patients diagnosed in stage 1 CKD and a good proportion of cats diagnosed in stage 2 CKD have plasma creatinine concentrations within the laboratory reference interval, the diagnosis of CKD being made on the basis of other clinical and laboratory findings. The majority of these patients will have plasma phosphate concentrations below the upper limit of the IRIS target range for their stage (see Table 1). Plasma concentrations of intact FGF23 could be used to help determine which of these cats will benefit from dietary phosphate restriction because they have evidence of mineral and bone disturbance. Furthermore, taking a baseline measurement of plasma FGF23 in these patients makes it possible to assess their response to treatment. Geddes et al., (2013b) showed that azotaemic normophosphataemic cats when transferred onto a phosphate restricted diet had a reduction in plasma FGF23 concentration 4 to 8 weeks later.
Table 1: Target ranges for serum phosphate by IRIS CKD stage
| IRIS CKD stage | Target serum phosphate concentration range |
|---|---|
| Stage 1 | 2.8 to 4.5 mg/dl (0.9 to 1.45 mmol/l) |
| Stage 2 | 2.8 to 4.5 mg/dl (0.9 to 1.45 mmol/l) |
| Stage 3 | 2.8 to 5 mg/dl (0.9 to 1.6 mmol/l) |
| Stage 4 | 2.8 to 6 mg/dl (0.9 to 1.9 mmol/l) |
It is important to assess plasma calcium concentration (ideally ionised calcium) in all cats with CKD at diagnosis of CKD as idiopathic hypercalcaemia has been associated with CKD (van den Broek et al., 2017). Some cats tend to develop hypercalcaemia when fed a phosphate restricted diet (Geddes et al., 2021), particularly if a markedly phosphate restricted diet is introduced in cats with relatively low plasma phosphate concentrations (Tang et al., 2021). Those cats which respond to phosphate restriction with an increase in plasma calcium concentration seem to demonstrate an increase rather than a reduction in plasma FGF23 concentration, emphasising again the importance of monitoring the response of dietary changes to both phosphate and calcium.
In CKD patients where plasma phosphate concentration is outside the IRIS target range for their IRIS stage (see Table 1), there is no need to measure plasma FGF23 concentration as plasma phosphate can be used to monitor response to treatment. Once plasma phosphate concentration falls into the target range for the stage of CKD, measurement of plasma FGF23 concentration would then be valuable to continue to monitor the response to treatment. If FGF23 plateaus well outside the recommended concentration, then additional measures to restrict intestinal phosphate absorption should be instituted (e.g. change to a more phosphate restricted renal diet or use of intestinal phosphate binding agents). In human medicine, meta-analyses of the available clinical trial data demonstrated that non-calcium containing phosphate binders reduced plasma FGF23 concentration whereas calcium containing phosphate binders proved less effective (Takkavatakarn et al., 2022; Zhao et al., 2022). Therefore, it seems prudent to recommend the use of non-calcium containing phosphate binders for veterinary patients (such as aluminium or lanthanum containing phosphate binders).
There are other reasons why FGF23 may be elevated in CKD patients that are not directly related to phosphate balance and that should be considered in patients with persistently elevated FGF23 where plasma phosphate has been controlled to within the IRIS target range. These include:
- Hypomagnesaemia exacerbates bone mineral disturbance and enhances FGF23 secretion even in normophosphataemic cats. Theoretically, supplementation of the diet with magnesium could be used to manage hypomagnesaemia in such cases although we are currently lacking evidence on which to base the safety and efficacy of this approach in veterinary patients
- Chronic inflammatory disease can contribute to activation of osteocytes by circulating cytokines and lead to increased plasma concentrations of FGF23. If it is possible to manage specific causes of chronic inflammation (e.g. treatment of periodontal disease) this may benefit the control of mineral bone disturbance in CKD. Evidence of the risk benefit of this approach in veterinary patients is currently lacking and such an approach would need to be assessed on an individual patient basis (e.g. the welfare benefit from treating dental disease is balanced against the risk of acutely exacerbating the CKD as a result of undertaking the treatment).
- Iron deficiency can exacerbate the severity of mineral bone disorder associated with CKD as discussed above. Iron deficiency can occur as a result of chronic inflammation (upregulation of hepcidin and impairment of iron recycling) and contribute to the anaemia of CKD with all three factors impacting on regulation of FGF23 synthesis and secretion (see Babitt & Sitara 2019). Accurate assessment of iron status in CKD patients is difficult even when anaemia occurs because the laboratory tests are hard to interpret definitively. Furthermore, the formulation of iron used for replacement therapy should be carefully considered based on the experience in human medicine, where some preparations for parenteral use increase intact FGF23 secretion (see Babitt & Sitara 2019).
6. Interpretation of plasma intact FGF23 concentrations
The current recommendations for plasma intact FGF23 concentrations are based on published data in cats diagnosed with early (non-azotaemic) CKD. These cases of early CKD (IRIS Stage 1 and a proportion of IRIS Stage 2 cats where CKD has been diagnosed despite serum creatinine being within the laboratory reference interval) have been identified either on the basis of developing azotaemic CKD within 12 months of screening (Finch et al., 2013) or because of elevated SDMA (>14 µg/dl; Sargent et al., 2019). These recommendations are also based on the physiological changes in FGF23 seen when dietary phosphate intake is increased from a low to a moderate level in healthy adult cats where long-term feeding of the moderately phosphate supplemented diet had no adverse effects on renal health (Coltherd et al., 2021). In this latter study, the plasma FGF23 response to increased phosphate intake rarely exceeded 400 pg/ml and once the cats has been eating the diets for 2-3 months, plasma FGF23 concentration was below 300 pg/ml in about 90% of cats.
In cases where CKD is diagnosed where plasma creatinine is within the laboratory reference interval, if plasma FGF23 concentration is >400 pg/ml, treatment with an early renal diet (moderately phosphate restricted) would be appropriate. If plasma FGF23 concentration is <300 pg/ml then there is no evidence of mineral bone disturbance in this cat and so no need to implement dietary phosphate restriction and if plasma FGF23 is between 300 and 400 pg/ml the cat should be monitored over time. These recommendations are based on the use of an ELISA assay for intact human FGF23 manufactured by Kainos Ltd., Japan and currently offered as a veterinary diagnostic test by IDEXX Laboratories and are summarised in Figure 1.
Figure 1: Flow chart indicating the utility of serum FGF23 in guiding treatment of early stage CKD cats
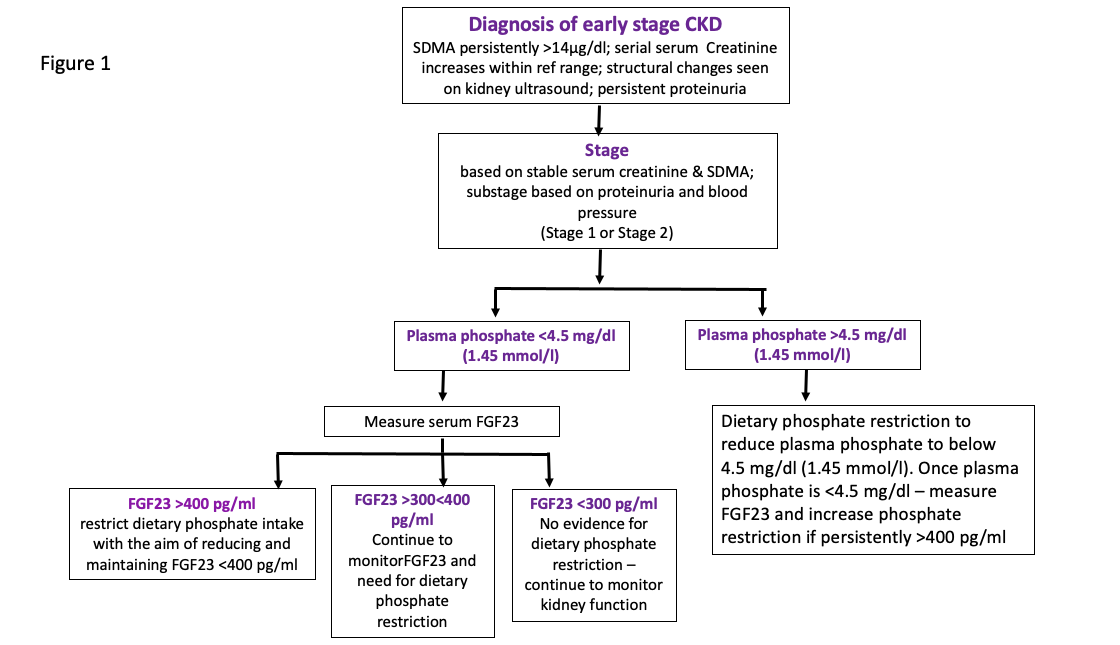
Recommendations for the use of FGF23 in monitoring the response of azotaemic CKD cats to phosphate restriction are currently empirical and based on the epidemiological study demonstrating a relationship between FGF23 and survival. The lowest quartile of cats (based on their plasma FGF23 concentration) at diagnosis of their CKD had the longest survival and these cats had plasma FGF23 concentrations of <700 pg/ml. Empirically, it would seem appropriate to suggest that FGF23 should be reduced below 700 pg/ml. That might only be achievable in stage 2 cats and a minority of stage 3 cats without the addition of intestinal phosphate binding agents and certainly only once plasma phosphate concentration is well within the target range. In addition, it may take a long time to reduce plasma FGF23 to its lowest concentration (weeks to months) with continual dietary phosphate restriction +/- intestinal phosphate binding agents. These recommendations are summarised in Figure 2.
Figure 2: Flow chart indicating the utility of measuring serum FGF23
in guiding the treatment of azotaemic CKD cats
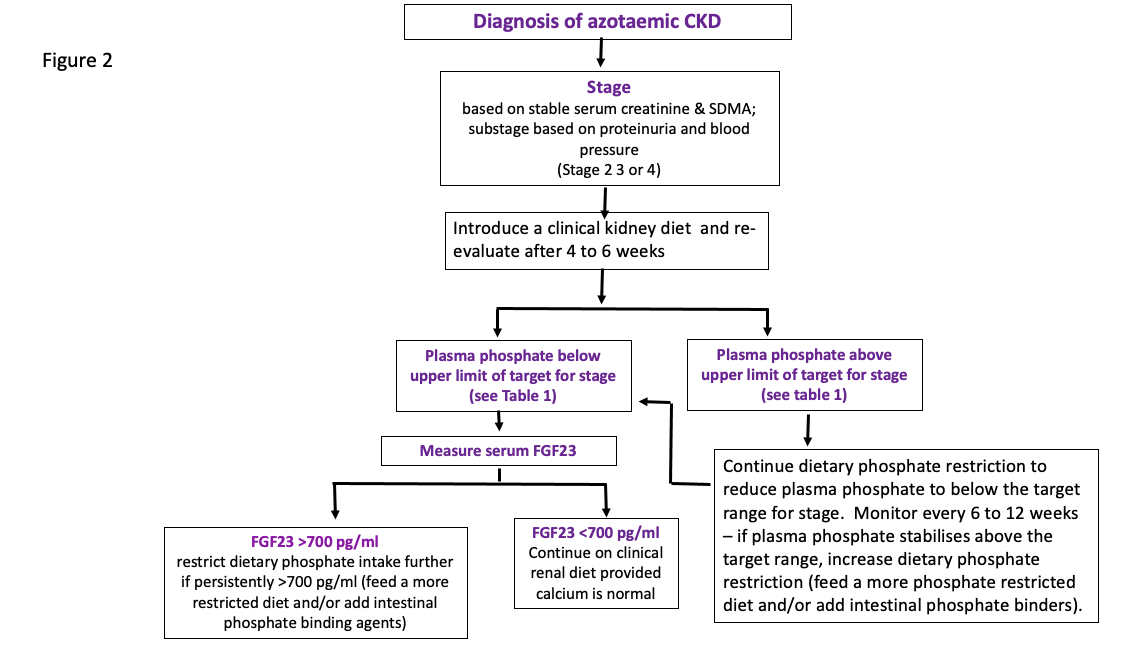
In the fullness of time, it is possible IRIS will have the evidence on which to base target FGF23 plasma concentrations for each stage of CKD for cases where plasma phosphate has been effectively controlled to within the current target ranges. This will require a larger number of cats to be monitored over time to assess the effect of treatment on FGF23 and to determine how this predicts outcome.
At the present time, it is not possible to make similar recommendations for dogs as the data are not available in the literature on which to base such recommendations.
7. Adverse effects of FGF23
One mechanism whereby FGF23 may harm the kidney and lead to tubular stress is through its physiological role of inhibiting phosphate reabsorption in the proximal convoluted tubule. Human subjects and cats consuming prepared foods supplemented with highly bioavailable forms of phosphate (e.g. sodium dihydrogen phosphate) will have a post-prandial peak in plasma phosphate concentration and increase in CPP formation (Tiong et al., 2022). Phosphate and the smallest CPPs (CPP monomers) are freely filtered. Both FGF23 and PTH will reduce the proportion of the filtered phosphate that is reabsorbed in the proximal convoluted tubule. This leads to a rise in tubular fluid phosphate concentration by the S3 segment of the tubule and the formation of larger CPPs which bind to TLR4 receptors on the tubular epithelium. The receptor and particles are internalised and this triggers tubular stress. Persistently high S3 tubular phosphate concentration (≥3 mmol/l) is associated with interstitial inflammation and fibrosis in laboratory animal models (Shiizaki et al., 2021). Theoretical calculations suggest such concentrations do occur in the last part of the proximal tubule when high levels of soluble phosphate are fed to cats, which has been associated with damage to renal health (Alexander et al., 2019; Elliott & Geddes 2022).
Whether or not FGF23 has a direct detrimental effect on the kidney once plasma concentrations start to increase in CKD patients remains to be determined. There is evidence that FGF23 can be directly responsible (even in the absence of klotho) for some of the extra-renal adverse effects of mineral bone disorder that accompany CKD in human patients and experimental models. There are clinical trials where therapies that inhibit FGF23 (e.g. blocking antibodies such as Burosumab) have proved effective in genetic disorders or tumours leading to excess FGF23 secretion (Insogna et al., 2018). These therapies are currently being studied to determine their benefit in CKD models before moving into human clinical CKD patients.
Once klotho deficiency occurs in the CKD patient and ever-increasing concentrations of FGF23 are produced by bone, there is clear evidence (from in vitro studies and laboratory animal studies) that FGF23 contributes to cardiac hypertrophy associated with CKD and vascular calcification. Both of these effects contribute to the cardiovascular disease which is often the cause of death in CKD human patients maintained on dialysis. These effects appear to be independent of klotho and, there is strong evidence cardiac muscle hypertrophy is driven by FGF23 activation of the FGF-4 receptor, activation which is exacerbated by heparin administered routinely to chronic dialysis patients (Yanucil et al., 2022).
Our understanding of the direct adverse effects of FGF23 in veterinary patients is in its infancy. In cats, clinical evidence of cardiovascular disease is not well documented in CKD patients with the exception of systemic hypertension. Nevertheless, in one long-term longitudinal study of cats with CKD, 19% died or were euthanised because of a cardiovascular complication of their CKD (aortic thromboembolism, sudden collapse and death, congestive heart failure) (Elliott et al., 2000). Furthermore, FGF23 concentration tends to be higher and plasma magnesium concentration tends to be lower in cats with hypertension and CKD when compared to normotensive CKD feline patients (van den Broek, 2018). In addition, there appears to be a strong correlation between plasma FGF23 concentration and plasma aldosterone concentration in a number of species, including the cat (Radloff et al., 2021) suggesting a role for FGF23 in the pathophysiology of hypertension associated with feline CKD.
9. Conclusion
The discovery of FGF23 as a regulator of phosphate homeostasis has changed our thinking of the way the bone-kidney-intestinal network functions to regulate calcium and phosphate. The implications of FGF23 as a biomarker in CKD patients are beginning to be realised in veterinary medicine with commercially available assays coming to the market. At the present time plasma intact FGF23 seems to have a place in determining which cats with early CKD would benefit most from moderate phosphate restriction and in monitoring the response to diet therapy in cats where plasma phosphate concentration is within the IRIS target ranges. The potential for FGF23 as a therapeutic target is a future possibility as its central importance in the pathophysiology of progressive CKD and the extra-renal consequences of CKD-MBD are strongly suggested by mechanistic experimental animal studies.
References
ADHR Consortium, (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nature Genetics 26, 345-8.
Akiyama K.I., Miura Y., Hayashi H., Sakata A., Matsumura Y., Kojima M., Tsuchiya K., Nitta K., Shiizaki K., Kurosu H., Kuro-O M. (2020). Calciprotein particles regulate fibroblast growth factor-23 expression in osteoblasts. Kidney International 97, 702-712.
Alexander J, Stockman J, Atwal J, Butterwick R, Colyer A, Elliott D, Gilham M, Morris P, Staunton R, Renfrew H, et al., (2019) Effects of the long-term feeding of diets enriched with inorganic phosphorus on the adult feline kidney and phosphorus metabolism. British Journal of Nutrition 121, 249-269.
Babitt JL, Sitara D. (2019) Crosstalk between fibroblast growth factor 23, iron, erythropoietin, and inflammation in kidney disease. Curr Opin Nephrol Hypertens.; 28(4):304-310.
Bon N, Frangi G, Sourice S, Guicheux J, Beck-Cormier S, Beck L. (2018) Phosphate-dependent FGF23 secretion is modulated by PiT2/Slc20a2. Mol Metab.; 11:197-204.
Coltherd JC, Staunton R, Colyer A, Thomas G, Gilham M, Logan DW, Butterwick R, Watson P. (2019) Not all forms of dietary phosphorus are equal: an evaluation of postprandial phosphorus concentrations in the plasma of the cat. British Journal of Nutrition 121, 270-284.
Coltherd J.C., Alexander J.E., Pink C., Rawlings J., Elliott J., Haydock R., Carvell-Miller L.J., Biourge V.C., Molina L., Butterwick R., et al., (2021) Towards establishing no observed adverse effect levels (NOAEL) for different sources of dietary phosphorus in feline adult diets: results from a 7-month feeding study. British Journal of Nutrition 126, 1626-1641.
Delitsikou V, Jarad G, Rajaram RD, Ino F, Rutkowski JM, Chen CD, Santos CXC, Scherer PE, Abraham CR, Shah AM, Feraille E, Miner JH, de Seigneux S. (2020) Klotho regulation by albuminuria is dependent on ATF3 and endoplasmic reticulum stress. FASEB J. 2020 Feb;34(2):2087-2104.
Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int.; 94(2):315-325.
Elliott J, Geddes RF. (2022) New concepts in phosphorus homeostasis and its impact on renal health with particular reference to the cat. Vet J.; 283-284:105842.
Elliott J, Rawlings JM, Markwell PJ, Barber PJ. (2000) Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract.; 41(6):235-42.
Finch N.C., Geddes R.F., Syme H.M., Elliott J. (2013) Fibroblast growth factor 23 (FGF-23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med. 27, 227-33.
Geddes RF, Finch NC, Elliott J, Syme HM. (2013) Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med.; 27(2):234-41.
Geddes RF, Elliott J, Syme HM. (2013b) The effect of feeding a renal diet on plasma fibroblast growth factor 23 concentrations in cats with stable azotemic chronic kidney disease. J Vet Intern Med.; 27(6):1354-61.
Geddes RF, van den Broek DHN, Chang YM, Biourge V, Elliott J, Jepson RE. (2021) The effect of attenuating dietary phosphate restriction on blood ionized calcium concentrations in cats with chronic kidney disease and ionized hypercalcemia. J Vet Intern Med.; 35(2):997-1007.
Harjes LM, Parker VJ, Dembek K, Young GS, Giovaninni LH, Kogika MM, Chew DJ, Toribio RE. (2017) Fibroblast Growth Factor-23 Concentration in Dogs with Chronic Kidney Disease. J Vet Intern Med.; 31(3):784-790.
HYP Consortium (1995) A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995; 11(2):130-6.
Insogna KL, Briot K, Imel EA, Kamenický P, Ruppe MD, Portale AA, Weber T, Pitukcheewanont P, Cheong HI, Jan de Beur S, Imanishi Y, Ito N, Lachmann RH, Tanaka H, Perwad F, Zhang L, Chen CY, Theodore-Oklota C, Mealiffe M, San Martin J, Carpenter TO; AXLES 1 Investigators. (2018) A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial Evaluating the Efficacy of Burosumab, an Anti-FGF23 Antibody, in Adults With X-Linked Hypophosphatemia: Week 24 Primary Analysis. J Bone Miner Res.; 33(8):1383-1393.
Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int.; 79(12):1370-8.
Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK. 2005 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. American Journal of Physiology Gastrointestinal and Liver Physiology 289, G1036-42.
Krajisnik T., Björklund P., Marsell R., Ljunggren O., Akerström G., Jonsson K.B., Westin G., Larsson T.E. (2007) Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. Journal of Endocrinology 195, 125-31
Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., et al., (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45-51
Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K.P., Baum M.G., Schiavi S., Hu M.C., Moe O.W., Kuro-o M. 2006. Regulation of fibroblast growth factor-23 signaling by klotho. Journal of Biological Chemistry 281, 6120-3.
Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. 2010 PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. American Journal of Physiology Renal Physiology 299, F882-9.
Liao YL, Chou CC, Lee YJ. (2019) The association of indoxyl sulfate with fibroblast growth factor-23 in cats with chronic kidney disease. J Vet Intern Med.; 33(2):686-693.
Miyakawa H, Hsu HH, Ogawa M, Akabane R, Miyagawa Y, Takemura N. (2021) Association between serum fibroblast growth factor-23 concentration and development of hyperphosphatemia in normophosphatemic dogs with chronic kidney disease. J Vet Intern Med.; 35(5):2296-2305.
Rudinsky AJ, Harjes LM, Byron J, Chew DJ, Toribio RE, Langston C, Parker VJ. (2018) Factors associated with survival in dogs with chronic kidney disease. J Vet Intern Med.; 32(6):1977-1982.
Sakan H, Nakatani K, Asai O, Imura A, Tanaka T, Yoshimoto S, Iwamoto N, Kurumatani N, Iwano M, Nabeshima Y, Konishi N, Saito Y. (2014) Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One. 23;9(1):e86301.
Sargent H.J., Jepson R.E., Chang Y.M., Biourge V.C., Bijsmans E.S., Elliott J. (2019) Fibroblast growth factor 23 and symmetric dimethylarginine concentrations in geriatric cats. Journal of Veterinary Internal Medicine 33, 2657-2664.
Segawa H, Kawakami E, Kaneko I, Kuwahata M, Ito M, Kusano K, Saito H, Fukushima N, Miyamoto K. 2003 Effect of hydrolysis-resistant FGF23-R179Q on dietary phosphate regulation of the renal type-II Na/Pi transporter. Pflugers Archive 446, 585-92.
Shiizaki K., Tsubouchi A., Miura Y., Seo K., Kuchimaru T., Hayashi H., Iwazu Y., Miura M., Battulga B., Ohno N., et al., 2021 Calcium phosphate microcrystals in the renal tubular fluid accelerate chronic kidney disease progression. Journal of Clinical Investigation. 131, e145693.
Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Fujita T., Nakahara K., Fukumoto S., Yamashita T. (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. Journal of Bone and Mineral Research 19, 429-35
Smith E.R., Hewitson T.D., Jahnen-Dechent W. 2020. Calciprotein particles: mineral behaving badly? Current Opinion in Nephrology and Hypertension. 29, 378-386.
Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, Koller A, Nizet V, White KE, Dixon JE. (2014) Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A.; 111(15):5520-5
Takashi Y., Kosako H., Sawatsubashi S, Kinoshita Y, Ito N, Tsoumpra MK, Nangaku M, Abe M, Matsuhisa M, Kato S, Matsumoto T, Fukumoto S. (2019) Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proceeding National Academy of Sciences U S A. 116, 11418-11427.
Takkavatakarn K, Wuttiputhanun T, Phannajit J, Praditpornsilpa K, Eiam-Ong S, Susantitaphong P. (2022) Effectiveness of fibroblast growth factor 23 lowering modalities in chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol.; 54(2):309-321.
Tang PK, Geddes RF, Chang YM, Jepson RE, Bijsmans E, Elliott J. (2021) Risk factors associated with disturbances of calcium homeostasis after initiation of a phosphate-restricted diet in cats with chronic kidney disease. J Vet Intern Med.; 35(1):321-332.
Tiong MK, Cai MMX, Toussaint ND, Tan SJ, Pasch A, Smith ER. (2022) Effect of nutritional calcium and phosphate loading on calciprotein particle kinetics in adults with normal and impaired kidney function. Sci Rep. 2022 May 5;12(1):7358.
Van den Broek (2018) Mineral and bone disorder in cats with chronic kidney disease. PhD Thesis, University of London.
van den Broek DH, Chang YM, Elliott J, Jepson RE. (2017) Chronic Kidney Disease in Cats and the Risk of Total Hypercalcemia. J Vet Intern Med.; 31(2):465-475.
van den Broek DHN, Chang YM, Elliott J, Jepson RE. (2018) Prognostic importance of plasma total magnesium in a cohort of cats with azotemic chronic kidney disease. J Vet Intern Med.; 32(4):1359-1371.
Vervloet MG. (2022) Shedding Light on the Complex Regulation of FGF23. Metabolites. 28;12(5):401.
Wheeler JA, Clinkenbeard EL. (2019) Regulation of Fibroblast Growth Factor 23 by Iron, EPO, and HIF. Curr Mol Biol Rep.; 5(1):8-17.
Wolf MT, An SW, Nie M, Bal MS, Huang CL. (2014) Klotho up-regulates renal calcium channel transient receptor potential vanilloid 5 (TRPV5) by intra- and extracellular N-glycosylation-dependent mechanisms. J Biol Chem.; 289(52):35849-57.
Yamamoto H, Ramos-Molina B, Lick AN, Prideaux M, Albornoz V, Bonewald L, Lindberg I. (2016) Posttranslational processing of FGF23 in osteocytes during the osteoblast to osteocyte transition. Bone.; 84:120-130.
Yanucil C, Kentrup D, Campos I, Czaya B, Heitman K, Westbrook D, Osis G, Grabner A, Wende AR, Vallejo J, Wacker MJ, Navarro-Garcia JA, Ruiz-Hurtado G, Zhang F, Song Y, Linhardt RJ, White K, Kapiloff MS, Faul C. (2022) Soluble α-klotho and heparin modulate the pathologic cardiac actions of fibroblast growth factor 23 in chronic kidney disease. Kidney Int.; 102(2):261-279.
Young GH, Wu VC. (2012) KLOTHO methylation is linked to uremic toxins and chronic kidney disease. Kidney Int. 2012 Apr;81(7):611-2.
Zhang R, Wang SY, Yang F, Ma S, Lu X, Kan C, Zhang JB (2021) Crosstalk of fibroblast growth factor 23 and anemia-related factors during the development and progression of CKD (Review). Exp Ther Med.; 22(4):1159.
Zhao SJ, Wang ZX, Chen L, Wang FX, Kong LD. (2022) Effect of different phosphate binders on fibroblast growth factor 23 levels in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Ann Palliat Med.; 11(4):1264-1277.